EN 1276 test – bacterial activity test on chemical disinfectants and antiseptics
This test method is said to be a suspension test method, and it is for testing chemical antiseptic and disinfectants for bactericidal activities on them. This method takes about four to five days to complete, and it is a slow process, so it needs a lot of time for the bacterial development for the different testing processes. Improper growth of bacteria may change the significant result of the test. This test can use nine other bacterias, and some additional strains can also work with it. All testing methods help conduct and evaluate bacterial activity on different tests with products like commercial disinfectants and antiseptics. This EN 1276 test is a kind of antibacterial test, and many similar tests are available for other products. The main aim is to test the disinfectant, antiseptics, and similar formulations against various bacterias. This test will help find the product’s capability with different real-time bacterias.
Antibacterial te
sting
These antibacterial testing methods help find the immunity and withstanding strength of the antibacterial layers and other similar products. And these tests are helpful to increase the abilities of customer products. In laboratories, they use various real-time bacterias as bacteria strains to test all the customer products. Each testing method will have different testing processes with several steps. Those antibacterial testing methods are
- ASTME2149
- AATCC-100
- JISZ2801
- JISL1902
- EN 1276
These are the antibacterial testing methods that are for various kinds of ways. Each testing is for different types of products because each product will differ from one another. These antibacterial testing methods can also process with various additional strains or bacterias, and these are the primary features of antibacterial testing methods. The EN 1276 test method is for testing disinfectants and antiseptics.
About EN 1276 testing
This testing method evaluates the bacterial activity on disinfectants and antiseptics for food, domestic, institutional and industrial uses. This EN 1276 test method involves various functions or processes to create the final output. It uses multiple organisms like Staphylococcus aureus (ATCC 6538p), Pseudomonas-Aeruginosa (ATCC 15442), Enterococcus (ATCC 10541) and Escherichia coli (ATCC 8739).
All these organisms help create various bacteria-based tests on customer products. And if the customer needs additional strains than these organisms, they can add some different organisms to the testing process. Each testing process will have further steps and preparation methods. All the steps are pre-planned and properly organized. Every bacteria and virus needs some specific environment to develop in the lab people create this environment to develop these bacterias and viruses.
Testing method for EN 1276
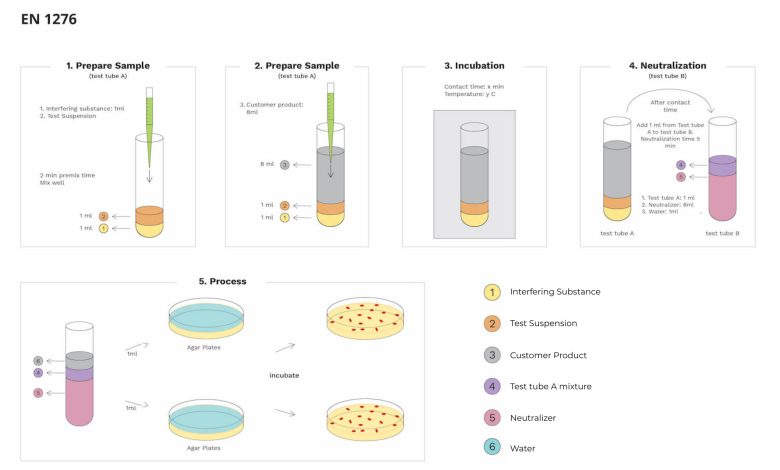
This test involves five steps of the process, and each step will have specific conditions and rules. And in every part of the process, some extra external chemicals and features are added with the primary sample. In this process, several components like interfering substance, test suspension, customer product, test tube A mixture, Neutralizer, and water are the essential components. Each component is part of a testing process. This test involves various techniques like
- Sample preparation I
- Sample preparation II
- Incubation
- Neutralization
- Processing
These are the steps involved with the antibacterial EN 1276 test with various organisms for various food, industry, institutional, domestic disinfectants and antiseptics. By completing all the processes, the final result will be from comparing the growth and activities of bacteria on different time bases.
Sample preparation I
The preparation of sample I starts with a clean and neet test tube, adding 1ml of interfering substance and 1ml of test suspension. After adding all the solutions, premix the total solution for two minutes, and after completing it, allow it to rest in that same test tube for some time. This first step of sample preparation works with the sample of the customer product to initiate the process of testing. These are the initial process available in the first sample preparation.
Sample preparation II
After completing the first sample preparation, the second preparation starts. With the same test tube A, add 8ml of customer product in this process. This process will be the second important step because it involves the customer product that needs proper testing. After adding the customer solution to test tube A, it will start its reaction with the previous solutions on test tube A. after this process test tube will move to the following processes.
Incubation
The incubation process is nothing but isolating the test tube from all kinds of physical and chemical disturbances and allowing it to settle in a particular environmental setup. This incubation process will take place for a specific time, and the period is known as contact time. Then this test tube is a particular temperature as per the requirement of the product and its strains to develop. These are the basic process that takes place in incubation. And then, the test tube moves to the following procedure.
Neutralization
The neutralization process involves two different test tubes. The first test tube is A, and the second test tube is B. Test tube A contains the customer solution and interfering substance & test suspensions. Test tube B will get 1 ml of test tube A solution and 8ml of Neutralizer in this neutralization process. It also contains 1ml of water which make the circle complete. This process takes place after the completion of contact time. These are the process of neutralization. And the total time for neutralization is about 5 minutes which will further make the customer product more reactive with bacterias.
Processing
After completing all the processes, the final step is processing. Only test tube B is required in this step and not test tube A. One ml of test tube B solution to two agar plates. And each dish will have one ml of solution. Then again, those two agar plates go for incubation for a specific time and along with a similar temperature. It is the final process in this EN 1276 test. After completing this, both the plates will undergo a micro examination to check the conditions of the customer product. Most processing processes will be the same for bacterial testing, and only some will have changes.
Test report for EN 1276 test
After completing all the processes, the test result will be by comparing the damage level to the product or by the withstanding capacity of the product against the bacterial strains. Each strain will react differently, and every product will have different results. While comparing with other test methods, this is a suspended test method, and it needs more waiting time than any other test method.
The ratio of damage or the growth between the bacterias in the two agar plates will determine the final result of the process. Each container will have a different count of organisms and different damage levels. All the procedures in this test will lead to the end of the process to find the outcome, and the primary motto of this EN 1276 test is to find the bacterial activity on the customer products. The final report for this test will explain the effectiveness of the customer product.
Testing methods
These testing methods are very interactive and efficient in their work. Each test will tell about the ability, immunity power and strength against chemical reactions of the customer products. These tests are the safety measures to destroy the community spread of bacterias and viruses through day-to-day products. These are the primary feature that every testing will fulfil.
Also read: EN 14476 – antiviral test on sanitizers and disinfectants





